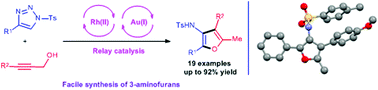
Facile synthesis of substituted 3-aminofurans through a tandem reaction of N-sulfonyl-1,2,3-triazoles with propargyl alcohols†
Abstract
A relay catalysis strategy for substituted 3-aminofurans synthesis has been developed. This transformation involves a tandem reaction sequence through aza-vinyl-rhodium(II) carbene O–H bond insertion, thermal propargyl-Claisen rearrangement and gold(I)-catalyzed intramolecular cyclization. More importantly, the current strategy employs simple feedstocks as starting materials, providing substituted 3-aminofurans in a highly efficient manner.

 Please wait while we load your content...
Please wait while we load your content...