Novel strategies for the synthesis of unsymmetrical glycosyl disulfides†
Abstract
Novel strategies for the efficient synthesis of unsymmetrical glycosyl disulfides are reported. Glycosyl disulfides are increasingly important as glycomimetics and molecular probes in glycobiology. Sialosyl disulfides are synthesised directly from the chlorosialoside Neu5Ac2Cl, proceeding via a thiol-disulfide exchange reaction between the sialosyl thiolate and symmetrical disulfides. This methodology was adapted and found to be successfully applicable to the synthesis of unsymmetrical glucosyl disulfides under mild conditions.
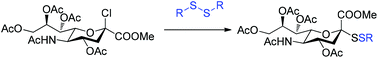

 Please wait while we load your content...
Please wait while we load your content...