Synthesis of α,ω-polyfluorinated α-amino acid derivatives and δ,δ-difluoronorvaline†
Abstract
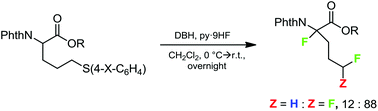
Intending to synthesize ω,ω-difluoroalkyl amino acid derivatives by oxidative desulfurization–fluorination reactions of suitable arylthio-2-phthalimido butanoates and pentanoates, in addition to small amounts of the target products, mainly α,ω-polyfluorinated amino acid derivatives were formed by additional sulfur-assisted α-fluorination. This novel structural motif was verified spectroscopically as well as by X-ray analysis. A plausible mechanism of formation is suggested. Using a different approach, δ,δ-difluoronorvaline hydrochloride was synthesized with at least 36% enantiomeric excess via deoxofluorination of the corresponding aldehyde.

 Please wait while we load your content...
Please wait while we load your content...