Rapid and efficient synthesis of α(1–2)mannobiosides†
Abstract
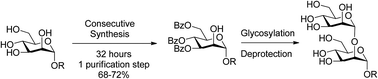
α(1,2)mannobiosides with different substituents at the reducing end have been synthesized by a common strategy using benzoyls as the permanent protecting groups and an acetyl as the orthogonal protecting group at position C2 of the glycosyl acceptor. The new synthetic strategy has been performed remarkably reducing the number of purification steps, the time of synthesis (less than 72 hours) and improving the overall yield at least three times with respect to the best procedure described in the literature at the moment. Additionally, this protecting group strategy is compatible with the presence of azido groups and the use of Cu catalyzed azide alkyne cycloaddition (CuAAC) also called “click chemistry” for conjugating the α(1–2)mannobiosides to different scaffolds for the preparation of mannosyl multivalent systems.

 Please wait while we load your content...
Please wait while we load your content...