Synthesis of hydrazinoheterocycles from Morita–Baylis–Hillman adducts of nitroalkenes with azodicarboxylates†
Abstract
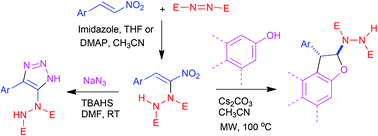
Conjugated nitroalkenes and nitrodienes undergo smooth α-hydrazination with azodicarboxylates through an imidazole catalyzed carbon–heteroatom bond formation under Morita–Baylis–Hillman conditions. The resulting hydrazinonitroalkenes take part in 1,3-dipolar cycloaddition with azide under mild conditions to give hydrazinotriazoles. A [3 + 2] annulation with phenols and naphthols involving Michael addition and cyclization as the key steps lead to arenodihydrofurans bearing a key hydrazinodicarboxylate moiety. Both regioisomers of naphthodihydrofurans could be synthesized by our methodology by employing the appropriate naphthol.

 Please wait while we load your content...
Please wait while we load your content...