Synthesis and characterization of chiral aza-macrocycles and study of their enantiomer recognition ability for organo-phosphoric acid and phosphonic acid derivatives by 31P NMR and fluorescence spectroscopy†
Abstract
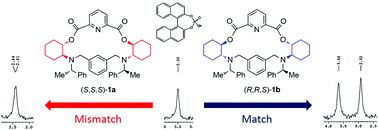
Two diastereomers of optically active N,O-containing new macrocycles with dual chirality of the ring and pendent group were synthesized and characterized. The difference in the accessibility of the cavity was explored to discriminate the enantiomers of the derivatives of organo-phosphoric and phosphonic acids by 31P NMR and fluorescence spectroscopy.

 Please wait while we load your content...
Please wait while we load your content...