Synthesis of new dicinnamoyl 4-deoxy quinic acid and methyl ester derivatives and evaluation of the toxicity against the pea aphid Acyrthosiphon pisum†
Abstract
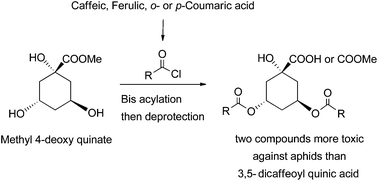
New dicinnamoyl (caffeoyl, feruloyl, ortho and para-coumaroyl) 4-deoxyquinic acid and esters were synthesized by using a new 4-deoxy quinic acid triol intermediate. The optimisation of both coupling and deprotection steps allowed the preparation in good yields of the target products either as the carboxylic acid or the methyl ester form. Eight new compounds were evaluated for their ability to influence the feeding behaviour of the pea aphid Acyrthosiphon pisum. Artificial diet bioassays showed that two compounds are toxic (mortality and growth inhibition) at lower concentrations than the reference 3,5-dicaffeoyl quinic acid.

 Please wait while we load your content...
Please wait while we load your content...