Direct synthesis of C-glycosides from unprotected 2-N-acyl-aldohexoses via aldol condensation–oxa-Michael reactions with unactivated ketones†
Abstract
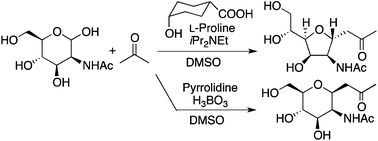
C-glycosides are important compounds as they are used as bioactive molecules and building blocks. We have developed methods to concisely synthesize C-glycosides from unprotected 2-N-acyl-aldohexoses and unactivated ketones; we designed aldol-condensation–oxa-Michael addition reactions catalyzed by amine-based catalysts using additives. Depending on the conditions used, C-glycosides were stereoselectively obtained. Our methods allowed the C–C bond formations at the anomeric centers of unprotected carbohydrates under mild conditions to lead the C-glycosides in atom- and step-economical ways.

 Please wait while we load your content...
Please wait while we load your content...