Readily prepared inclusion forming chiral calixsalens†
Abstract
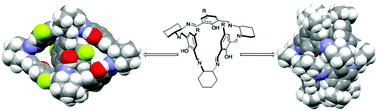
Calixsalens, chiral triangular hexaimines are readily synthesized by [3 + 3] cyclocondensation of trans-(R,R)-1,2-diaminocyclohexane with 2-hydroxyisophthalaldehyde derivatives. The usually rigid calixsalen ring is able to invert its conformation as a consequence of steric repulsion between bulky substituents at the C5 positions of the aromatic rings. The steric and electronic nature of the substituents does not affect only the conformation of the macrocycle. Small polar substituents enforce dimeric self-association to form an apohost where each of the monomers simultaneously serves as the host and the guest of its partner. Non-associating calixsalens form assemblies in which two symmetry-related molecules are arranged in a head-to-head fashion to form a capsule, or unimolecular cages that are able to entrap solvent molecules in their intrinsic voids.

 Please wait while we load your content...
Please wait while we load your content...