Facile synthesis of the NNRTI microbicide MC-1220 and synthesis of its phosphoramidate prodrugs
Abstract
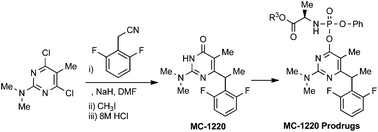
A facile and novel synthetic route to MC-1220 was achieved by condensation of 4,6-dichloro-N,N-5-trimethylpyrimidin-2-amine (1) with the sodium salt of 2,6-difluorophenylacetonitrile, followed by methylation and strong acidic hydrolysis. The prodrugs of MC-1220 were synthesized by reaction of chlorophosphoramidate derivatives (7a–e) or α-acetobromoglucose with the sodium salt of MC-1220. The stability and anti-HIV-1 activity of phosphoramidate prodrugs turned out to be comparable to those of the parent drug MC-1220.

 Please wait while we load your content...
Please wait while we load your content...