Fabrication of amorphous CoMoS4 as a bifunctional electrocatalyst for water splitting under strong alkaline conditions†
Abstract
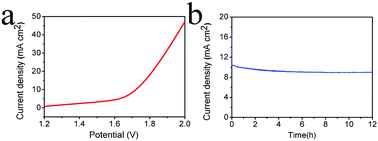
With the socio economic development, people have paid more and more attention to energy source problems, especially to clean and renewable energy such as hydrogen. It is appealing but still challenging to find or design an appropriate catalyst which is inexpensive and efficient for both the hydrogen evolution reaction (HER) and oxygen evolution reaction (OER) in the same electrolyte. In this work, we develop a facile synthesis of amorphous defect-rich CoMoS4via a one-step hydrothermal method, and under alkaline conditions; the CoMoS4 electrode can generate a current density of 10 mA cm−2 at the overpotentials of 143 mV for HER and 342 mV for OER in 1.0 M KOH, respectively. A cell voltage of 1.72 V is required to achieve a current density of 10 mA cm−2 with long-term stability in an electrolyzer using the CoMoS4/CC electrode as both the anode and cathode.

 Please wait while we load your content...
Please wait while we load your content...