Highly active nickel–cobalt/nanocarbon thin films as efficient water splitting electrodes†
Abstract
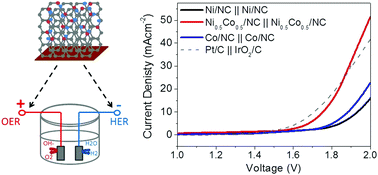
Developing low cost, highly active and stable electrocatalysts for both the hydrogen evolution reaction (HER) and the oxygen evolution reaction (OER) using the same electrolyte has remained a major challenge. Herein, we report a novel and robust material comprised of nickel–cobalt nanoparticles coated on a porous nitrogen-doped carbon (NC) thin film synthesized via a two-step pulsed laser deposition technique. The optimized sample (Ni0.5Co0.5/NC) achieved the lowest overpotentials of 176 mV and 300 mV at a current density of 10 mA cm−2 for HER and OER, respectively. The optimized OER activity might be attributed to the available metal oxide nanoparticles with an effective electronic structure configuration and enhanced mass/charge transport capability. At the same time, the porous nitrogen doped carbon incorporated with cobalt and nickel species can serve as an excellent HER catalyst. As a result, the newly developed electrocatalysts manifest high current densities and strong electrochemical stability in overall water splitting, outperforming most of the previously reported non-precious metal-based catalysts.


 Please wait while we load your content...
Please wait while we load your content...