Water bridge coordination on the metal-rich facets of Gd2O3 nanoplates confers high T1 relaxivity†
Abstract
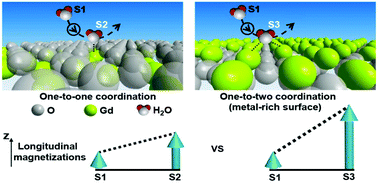
The realization of the nature of water coordination on the solid surfaces may provoke an essential understanding of T1 relaxation enhancement, especially in nanoparticulate systems. We report herein that the T1 relaxivity of Gd2O3 nanoplates is highly dependent on water coordinating behaviors on different surfaces. Gd2O3 nanoplates with metal-rich {100} facets showed an approximately 4-fold higher r1 value compared to that with oxygen-terminated {111} facets. Density functional theory (DFT) calculations show that the enhanced T1 relaxivity of Gd2O3 {100} nanoplates may be ascribed to the high density of accessible Gd3+, fast exchange of water, and more importantly, multicenter (one-to-two) coordination for water molecules with magnetic centers on the metal-rich surface.


 Please wait while we load your content...
Please wait while we load your content...