Precise redox-sensitive cleavage sites for improved bioactivity of siRNA lipopolyplexes†
Abstract
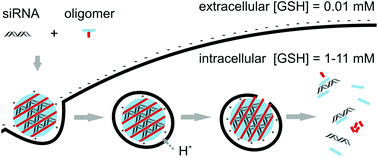
Lipo-oligomers have been proven as potent siRNA carriers based on stable electrostatic and hydrophobic complex formation and endosomal membrane destabilization. Although high stability of siRNA polyplexes is desirable in the extracellular space and cellular uptake, intracellular disassembly is important for the cytosolic release of siRNA and RNA-induced silencing complex formation. To improve the release, bioreducible sequence-defined lipo-oligomers were synthesized by solid-phase assisted synthesis using the disulfide building block Fmoc-succinoyl-cystamine for precise positioning of a disulfide unit between a lipophilic diacyl (bis-myristyl, bis-stearyl or bis-cholestanyl) domain and an ionizable oligocationic siRNA binding unit. Reducible siRNA polyplexes show higher gene silencing efficacy and lower cytotoxicity than their stable analogs, consistent with glutathione-triggered siRNA release and reduced lytic activity.

 Please wait while we load your content...
Please wait while we load your content...