Nanoscale stabilization of zintl compounds: 1D ionic Li–P double helix confined inside a carbon nanotube†
Abstract
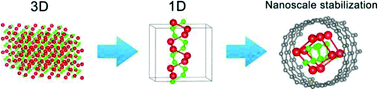
One-dimensional (1D) ionic nanowires are extremely rare materials due to the difficulty in stabilizing 1D chains of ions under ambient conditions. We demonstrate here a theoretical prediction of a novel hybrid material, a nanotube encapsulated 1D ionic lithium monophosphide (LiP) chain, featuring a unique double-helix structure, which is very unusual in inorganic chemistry. This nanocomposite has been investigated with density functional theory, including molecular dynamics simulations and electronic structure calculations. We find that the formation of the LiP double-helical nanowire is facilitated by strong interactions between LiP and CNTs resulting in a charge transfer. This work suggests that nanostructured confinement may be used to stabilize other polyphosphide 1D chains, thus opening new ways to study the chemistry of zintl compounds at the nanoscale.

 Please wait while we load your content...
Please wait while we load your content...