Phytocannabinoids: a unified critical inventory
Abstract
Covering up to January 2016
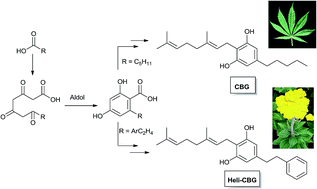
Cannabis sativa L. is a prolific, but not exclusive, producer of a diverse group of isoprenylated resorcinyl polyketides collectively known as phytocannabinoids. The modular nature of the pathways that merge into the phytocannabinoid chemotype translates in differences in the nature of the resorcinyl side-chain and the degree of oligomerization of the isoprenyl residue, making the definition of phytocannabinoid elusive from a structural standpoint. A biogenetic definition is therefore proposed, splitting the phytocannabinoid chemotype into an alkyl- and a β-aralklyl version, and discussing the relationships between phytocannabinoids from different sources (higher plants, liverworts, fungi). The startling diversity of cannabis phytocannabinoids might be, at least in part, the result of non-enzymatic transformations induced by heat, light, and atmospheric oxygen on a limited set of major constituents (CBG, CBD, Δ9-THC and CBC and their corresponding acidic versions), whose degradation is detailed to emphasize this possibility. The diversity of metabotropic (cannabinoid receptors), ionotropic (thermos-TRPs), and transcription factors (PPARs) targeted by phytocannabinoids is discussed. The integrated inventory of these compounds and their biological macromolecular end-points highlights the opportunities that phytocannabinoids offer to access desirable drug-like space beyond the one associated to the narcotic target CB1.

 Please wait while we load your content...
Please wait while we load your content...