Calyculin: Nature's way of making the sponge-derived cytotoxin
Abstract
Covering: up to 2015.
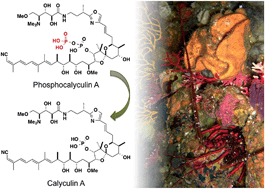
Calyculin A is a major cytotoxic compound isolated from the Japanese marine sponge Discodermia calyx. Its potent cytotoxicity is attributable to the specific inhibition of protein phosphatases 1 and 2A, as in the case of okadaic acid and the microcystins. Its chemical structure is well-designed not only for enzyme inhibition but also for higher membrane permeability in order to impart its potent cytotoxicity. The biosynthetic gene cluster of this densely functionalized polyketide and nonribosomal peptide hybrid molecule was recently identified from the sponge–microbe association. The producer organism and the dynamic bioconversion process were also revealed. In this highlight, we focus on the recent studies addressing nature's design and biogenesis of the sponge-derived cytotoxin, calyculin A.

 Please wait while we load your content...
Please wait while we load your content...