Crystal structure and physical properties of 1-methyl-3-(carboxymethyl)benzimidazolium betaine·CuBr2 in crystal and water solution†
Abstract
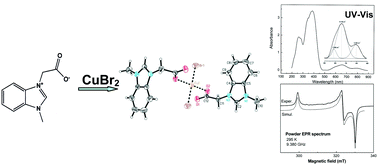
A new Cu(II) carboxylate coordinating compound [1-methyl-3-carboxymethyl benzimidazolium betaine]2CuBr2 was synthesized and crystallized. The crystal has the triclinic symmetry P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) , with unit cell dimensions a = 7.9693, b = 8.4129, c = 9.1302 Å, α = 68.058, β = 85.402 and γ = 71.258 deg. (Z = 1), and molecules stacked along the a-axis. Cu(II)-complexes are planar and four-coordinated with chromophore CuO2Br2, where two oxygen atoms belong to the carboxylate groups of two betaines acting as unidentate ligands. The compound was characterized by two-dimensional 1H and 13C NMR spectroscopy for the determination of the correlation between protons of a ligand molecule. NMR spectra confirm the coordination of Cu(II) ions and allow identification of H(2) proton as easily detached in basic conditions. FT-IR spectra confirm the unidentate coordination of the betaine carboxylate group. UV-Vis spectra show three bands in d–d-transition region. Energies of these transitions were used in the interpretation of the EPR results. From powder and single crystal EPR measurements the g-factors were determined as gx = 2.072, gy = 2.030, gz = 2.241. A non-typical g-factor sequence is a consequence of the orbital mixing in the ground state of Cu(II) complex of D2h symmetry. The g-factors were interpreted in terms of the Molecular Orbital (MO) theory which delivered the Cu(II) unpaired electron density delocalization onto the ligand molecules. A strong delocalization on betaine molecules via in-plane ground-state orbital was found and unexpectedly also via out-of plane orbital directed towards the non-coordinating oxygen of the betaine carboxylate group.
, with unit cell dimensions a = 7.9693, b = 8.4129, c = 9.1302 Å, α = 68.058, β = 85.402 and γ = 71.258 deg. (Z = 1), and molecules stacked along the a-axis. Cu(II)-complexes are planar and four-coordinated with chromophore CuO2Br2, where two oxygen atoms belong to the carboxylate groups of two betaines acting as unidentate ligands. The compound was characterized by two-dimensional 1H and 13C NMR spectroscopy for the determination of the correlation between protons of a ligand molecule. NMR spectra confirm the coordination of Cu(II) ions and allow identification of H(2) proton as easily detached in basic conditions. FT-IR spectra confirm the unidentate coordination of the betaine carboxylate group. UV-Vis spectra show three bands in d–d-transition region. Energies of these transitions were used in the interpretation of the EPR results. From powder and single crystal EPR measurements the g-factors were determined as gx = 2.072, gy = 2.030, gz = 2.241. A non-typical g-factor sequence is a consequence of the orbital mixing in the ground state of Cu(II) complex of D2h symmetry. The g-factors were interpreted in terms of the Molecular Orbital (MO) theory which delivered the Cu(II) unpaired electron density delocalization onto the ligand molecules. A strong delocalization on betaine molecules via in-plane ground-state orbital was found and unexpectedly also via out-of plane orbital directed towards the non-coordinating oxygen of the betaine carboxylate group.

 Please wait while we load your content...
Please wait while we load your content...