Rigid geometry 8-arylimino-7,7-dimethyl-5,6-dihydroquinolyl nickel bromides: single-site active species towards ethylene polymerization†
Abstract
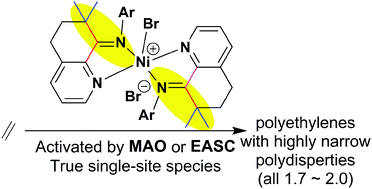
The fused ring heterocyclic ketone, 5,6-dihydro-7,7-dimethylquinolin-8-one, was prepared and employed for the synthesis of a series of 8-arylimino-7,7-dimethyl-5,6-dihydroquinoline derivatives (aryl = 2,6-Me2Ph (L1), 2,6-Et2Ph (L2), 2,4,6-Me3Ph (L3), 2,6-Et2-4-MePh (L4), 2,6-i-Pr2Ph (L5)). The reaction of L1–L4 with (DME)NiBr2 (DME = 1,2-dimethoxyethane) gave the corresponding cationic bis-chelates, [(Lx)2NiBr][Br] (Lx = L1 (Ni1), L2 (Ni2), L3 (Ni3), L4 (Ni4)), as bromide salts; no such complex could be isolated with the most sterically bulky L5. All new compounds were characterized using IR spectroscopy, elemental analysis and in the case of L1–L5 using 1H and 13C NMR spectroscopy. Furthermore, the molecular structures of Ni1 and Ni3 have been determined and they reveal cation–anion pairs based on a trigonal bipyramidal nickel-containing cation charge balanced by a bromide counterion. In addition, X-ray photoelectron spectroscopy (XPS) was used to probe the solid state structures of L1, L3 and Ni1–Ni4; this technique provided valuable information regarding the net charge on nickel within the complexes. Upon activation with either methylaluminoxane (MAO) or ethylaluminium sesquichloride (EASC), all nickel complexes exhibited high activities towards ethylene polymerization and produced polyethylene waxes with low molecular weights. The catalytic activities, Ni1 [2,6-di(Me)] > Ni3 [2,4,6-tri(Me)] > Ni4 [2,6-di(Et)-4-Me] > Ni2 [2,6-di(Et)], correlated well with the trend in net charges observed in XPS analysis. The polydispersities (1.7–2.0) obtained for polyethylenes are narrow and indicate genuinely single-site active species for these catalysts. These performance characteristics have been attributed to the influence of the rigid geometry imparted by L1–L5 that, due to the presence of 7,7-dimethyl-substituents, prevents imine–enamine tautomerization.


 Please wait while we load your content...
Please wait while we load your content...