Oxidative organophotoredox catalysis: a regioselective synthesis of 2-nitro substituted imidazopyridines and 3-substituted indoles, initiated by visible light†
Abstract
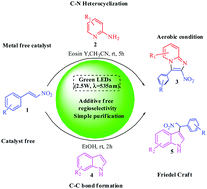
We have established a mild, metal-free, one pot, visible light-catalyzed procedure for a highly regioselective synthesis of 2-nitro-3-arylimidazo [1,2-a] pyridines via nitroalkene and 2-aminopyridine under an open atmosphere involving a photoredox catalyst, Eosin Y, which is an inexpensive organic dye that has been employed as a photo sensitizer in the conversion. This protocol serves as an example of green chemistry, due to the fact that molecular oxygen and visible light have been utilized effectively for the transformation. The procedure involves intramolecular C–N heterocyclization, followed by aerobic oxidation and C–C bond formation at room temperature. This green protocol has also been successfully extended to the regioselective synthesis of 3-substituted indoles via indole and nitroalkene by a free radical pathway.


 Please wait while we load your content...
Please wait while we load your content...