Chiral 1,2-dithiine as a sulfur rich electron acceptor†
Abstract
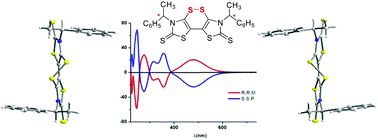
The selective synthesis of both enantiomers of a sulfur rich electron acceptor containing two 1-phenylethyl groups of the same chirality and a chiral axis is described. Cyclisation into enantiopure dithiine has been induced by the presence of a chiral substituent on the nitrogen atom of a thiazoline-2-thione dithiolate precursor.

 Please wait while we load your content...
Please wait while we load your content...