Enantioselective transfer hydrogenation, a key step for the synthesis of 3-aminotetrahydroquinolines†
Abstract
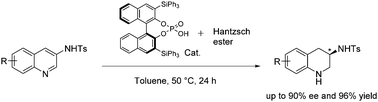
An enantioselective transfer hydrogenation has been successfully achieved to furnish 3-aminotetrahydroquinolines. The reaction was conducted in the presence of Hantzsch dihydropyridine and a catalytic amount of chiral phosphoric acid under mild conditions.

 Please wait while we load your content...
Please wait while we load your content...