Half-sandwich iridiumIII complexes with pyrazole-substituted heterocyclic frameworks and their biological applications†
Abstract
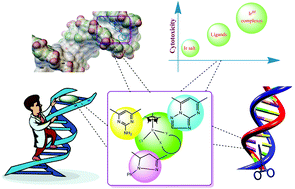
Low-spin IrIII organometallic half-sandwich complexes of type [(η5-C5Me5)Ir(XY)Cl]+ (XY = bipyrazoles (4a–4b)/pyrimidin-2-amines (5a–5b)/triazolo[1,5-a]pyrimidines (6a–6b)) have been synthesized and characterized. All the newly synthesized compounds have been evaluated for their DNA binding properties with calf thymus (CT DNA), which revealed enhancement in the binding constant (Kb) of the complexes. The compounds bearing an imidazole substituent proved to be better binders than compounds containing a phenoxy linkage. Molecular docking attests that π–π stacking interactions have been observed between the receptor and the compounds. Furthermore, the observed DNA cleavage potency has been ascribed to a multitarget mechanism of action of these compounds. Intriguingly, the chelation of ligands with IrIII led to a remarkable enhancement of antibacterial activity against the arbitrarily selected two Gram +ve and three Gram −ve bacterial strains. The complexes of triazolo[1,5-a]pyrimidines proved to be the most cytotoxic compounds towards brine shrimp and S. pombe cells compared to pyrazole-containing heterocyclic frameworks. All complexes showed potent cytotoxicity as compared to the ligands, with IC50 values ranging from 78 to 234 μM toward A549 human lung cancer cells. The potency of the compounds toward these cancer cells was in the order pyrimidin-2-amines > bipyrazoles > triazolopyrimidines.

 Please wait while we load your content...
Please wait while we load your content...