Competition and cooperativity of σ-hole and π-hole intermolecular interactions between carbon monoxide and bromopentafluorobenzene
Abstract
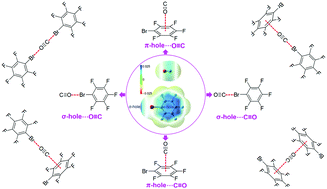
Theoretical investigations of the interactions between carbon monoxide (CO) and bromopentafluorobenzene (C6F5Br) have been carried out at both M06-2X/6-311++G(2d,2p) and M06-2X/aug-cc-pVTZ levels of theory. Because both C and O atom-ends of CO show negative electrostatic potential, they can favorably interact with the positive electrostatic potential generated by the σ-hole of Br and the π-hole of the aromatic ring of C6F5Br, yielding four different dimers and trimers. Their structures, spectroscopy and nature were systematically studied. The competition between the interactions involving the σ-hole of the Br atom and the π-hole of C6F5Br with the C and O atom-ends of CO was illustrated for the dimers and trimers, and meanwhile, the cooperativity between the two components of the trimers was elaborated. In addition, the experimental FT-IR and fluorescence spectra were measured for C6F5Br and the mixed C6F5Br and CO system without and with the solvent of hexane to gain information on the formation of molecular complexes between C6F5Br and CO. The results presented in this work are beneficial for the understanding of the competition and cooperativity of σ- and π-hole intermolecular interactions.

 Please wait while we load your content...
Please wait while we load your content...