The nephelauxetic effect of Eu(iii)–N-donor compounds probed using fluorescence spectroscopy—further evidence for covalence?†
Abstract
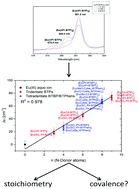
The 7F0 emission bands of Eu(III)–N-donor complexes are investigated using time resolved laser fluorescence spectroscopy (TRLFS) and fluorimetry. The observed bathochromic shifts resulting from the complexation of Eu(III) with N-donor ligands are considerably more pronounced than those observed for hard oxygen donor ligands. The magnitude of these shifts results from a strong nephelauxetic effect caused by the interaction of Eu(III) with N-donor ligands. Since the extent of the nephelauxetic effect is correlated with the degree of covalence in a metal–ligand bond, the results prove a significant share of covalence in the Eu(III)–N-donor bonds in addition to electrostatic interactions. Furthermore, a linear correlation between the shift of the 7F0 emission band of the Eu(III) complexes with BT(B)P/BTPhen ligands relative to the Eu(III) aquo ion and the number of N-donors in the first coordination sphere of Eu(III) is established: CNL = 0.109·Δν + 0.134, [Δν] = cm−1. Taking into account the known denticity of BTP/BTBP/BTPhen ligands the stoichiometry of the investigated complexes is easily determined by using the above equation.

 Please wait while we load your content...
Please wait while we load your content...