Chemical transformation of ginsenoside Re by a heteropoly acid investigated using HPLC-MSn/HRMS†
Abstract
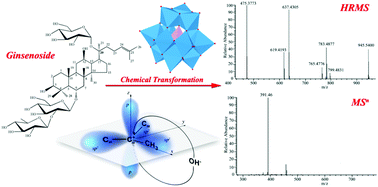
The potential of heteropoly acid H3PW12O40 to catalyze the chemical transformation of ginsenoside Re into rare ginsenosides was explored. This homogeneous catalyst can be recycled by extraction with diethyl ether. Eight resulting products were separated and identified through a developed high-performance liquid chromatography coupled with multistage tandem mass spectrometry and high-resolution mass spectrometry (HPLC-MSn/HRMS) method. Multistage tandem mass spectrometry was employed to trace the source of fragments and determine fragmentation pathways. Also, high-resolution mass spectrometry was used for the accurate structural elucidation of fragments. Ginsenosides 25-OH-Rg6 and 25-OH-F4, consisting of the aglycone structures of “3β, 12β, 25-trihydroxy-dammar-20 (21/22)-ene”, were obtained via chemical transformation for the first time. Chemical transformation pathways of ginsenoside Re were summarized, which involved deglycosylation, hydration, dehydration, and epimerization reactions. A carbenium ion mechanism was further employed to elucidate each transformation process, and the stability of carbenium ions was supposed to be responsible for the reaction pathways and selectivity.

 Please wait while we load your content...
Please wait while we load your content...