Design, synthesis and characterization of novel dioxime ligand-based cobaloxime compounds for application in the coupling of CO2 with epoxides
Abstract
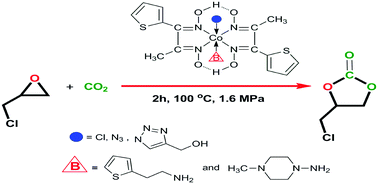
In this study, we introduce a simple and rapid two-step method for the synthesis of the monooxime (1) and dioxime ligand (LH2) (2) from 2-propanoyl thiophene as a ketone and its six novel cobaloxime complexes obtained by click chemistry (3–8), which were characterized by a range of spectroscopic techniques (1H- and 13C-NMR, FT-IR, and UV-Vis spectroscopy, LC-MS, and melting point and magnetic susceptibility measurements) as well as elemental analysis. The convenient synthesis of six new octahedral cobaloxime complexes of the general molecular formula [(LH)2Co(X)(B)] (where X = Cl, N3, or the 1,2,3-triazole ring and B = 1-amino-4-methylpiperazine (AMP) or 2-thiophene ethylamine (TEA) as an axial base) were conducted for the coupling of epoxides with the greenhouse gas CO2 to afford the corresponding five-membered cyclic carbonates under optimum conditions and without using any solvent. Furthermore, the effects of temperature, CO2 pressure and time on the yield of the five-membered cyclic carbonate were investigated systematically.

 Please wait while we load your content...
Please wait while we load your content...