Effect of configurational isomerism and polymorphism on chalcone fluorescent properties†
Abstract
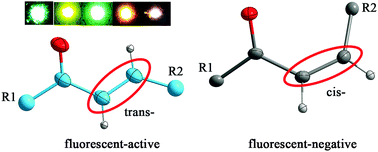
Three chalcones, 3-(9-anthryl)-1-(4-methoxyphenyl)prop-2-en-1-one (I), 3-(9-anthryl)-1-phenyl-prop-2-en-1-one (II) and 3-(1-pyrenyl)-1-(4-methoxyphenyl)prop-2-en-1-one (III), were synthesized and crystallized in different solvents to provide different forms such as two forms of I (Ia and Ib), four forms of II (IIa, IIb, IIc, and IId) and four forms of III (IIIa, IIIb, IIIc, and IIId). Single crystal X-ray diffraction was conducted on the samples except for IIa, IIIa, and IIIc, and powder X-ray diffraction, thermal behavior, solid-state absorption and fluorescence spectroscopy were investigated for all the crystals. It was found that these forms possess different configurational isomers and conformational polymorphs. Among them Ia, IId, IIIb and IIId possess the trans configuration, whereas Ib, IIb, and IIc show the cis configuration with a higher melting point than their trans isomers. These forms with trans configuration are fluorescent and IIIb has a new red shifted fluorescent peak relative to IIId, which can be explained by the strong slipped face-to-face π-stacked arrangement of the pyrene chromophores in IIIb. IId shows a new red shifted fluorescent peak relative to Ia, which can be explained by the overwhelming C(anthracene)–H⋯π(anthracene ring) interactions with an edge-to-face slipped π-stacked arrangement in IId. Although we were unable to obtain a single crystal of IIa, it can still be speculated that IIa possesses the trans conformation and has stronger interactions of anthracene fluorophores than IId by combining the two facts that IIa can be formed from IId by heating, which was tested by DSC and HSM and it has only one new red shifted fluorescent peak relative to Ia or IId.

 Please wait while we load your content...
Please wait while we load your content...