Nitrogen versus phosphorus nucleophiles – how changing the nucleophilic heteroatom affects ionic liquid solvent effects in bimolecular nucleophilic substitution processes†
Abstract
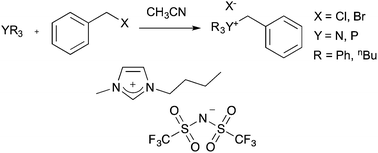
A series of nitrogen and phosphorus nucleophiles have been investigated to determine whether the previously established ionic liquid solvent effects on a bimolecular nucleophilic substitution (SN2) reaction vary with the nature of the nucleophilic centre. Reaction of group 15 triphenyl nucleophiles with benzyl bromide showed a different trend in the rate constant with increasing proportions of ionic liquid in the reaction mixture than was observed with pyridine. This result suggests additional interactions are important; a supposition supported by differences in reaction outcome observed when the electrophile was varied in reactions with triphenylphosphine. A novel ionic liquid solvent effect was observed in the reaction of tributylamine with benzyl bromide, with the position of equilibrium varying with the proportions of the ionic liquid present in the reaction mixture. Overall, the work presented demonstrates the importance of considering all possible interactions between an ionic liquid solvent and species along the reaction coordinate and has expanded upon our current predictive framework for ionic liquid solvent effects. Such understanding is important as it allows further development of a predictive framework for the application of ionic liquids in preparative chemistry.

 Please wait while we load your content...
Please wait while we load your content...