Organoselenium-catalyzed vicinal dichlorination of unsaturated phosphonates†
Abstract
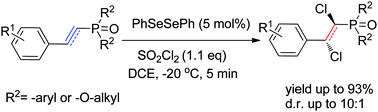
Diphenyl diselenide (PhSeSePh) was effective as a pre-catalyst for the vicinal dichlorination of α,β-unsaturated phosphonates with sulfuryl chloride. The reaction conditions were mild and the desired products were formed in up to 93% yield and moderate diastereoselectivity (10 : 1). The important diphenylselenium dichloride intermediate was obtained and characterized by X-ray crystallography.

 Please wait while we load your content...
Please wait while we load your content...