Total synthesis of Sceletium alkaloids (±)-joubertinamine, (±)-epijoubertinamine, (±)-tortuosamine and formal synthesis of (±)-mesembrine, (±)-N-formyltortuosamine†‡
Abstract
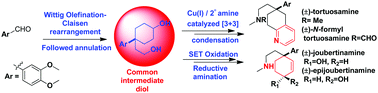
An efficient collective formal/total synthesis of Sceletium alkaloids and their seco-congeners has been reported. The bicyclic core of (±)-mesembrine, (±)-tortuosamine, and (±)-N-formyltortuosamine with all required functionalities including the characteristic quaternary benzylic carbon is achieved by employing the Wittig olefination–Claisen rearrangement protocol and synergistic Cu(I)/iminium catalyzed [3 + 3] condensation of O-acetylketoxime and α,β-unsaturated aldehyde (acrolein).

 Please wait while we load your content...
Please wait while we load your content...