Coordination compounds of a hydrazone derivative with Co(iii), Ni(ii), Cu(ii) and Zn(ii): synthesis, characterization, reactivity assessment and biological evaluation†
Abstract
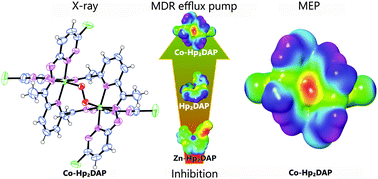
A new hydrazone-type ligand with a five N-donor set and its coordination compounds with Co(III), Ni(II), Cu(II) and Zn(II) metal centres were synthesized. The crystal and molecular structure of the Co(III) complex was determined by X-ray structural analysis. All the compounds were characterized by elemental analysis, molar conductivity and FT-IR spectral data. The cobalt(III) compound is a neutral binuclear complex formed by coordination of two, doubly deprotonated ligand anions as bridges between the Co(III) centres. The metal centres are additionally connected by a peroxido bridge, which was observed for the first time in Co(III) complexes with similar ligands. The other coordination compounds are mononuclear complexes wherein only one doubly deprotonated ligand is coordinated to the central ion in a tetradentate fashion. The structures were theoretically investigated employing density functional theory (DFT) calculations with B3LYP exchange–correlation functional and LACV3P+(d,p) basis sets for the coordination compounds and 6-31+G(d,p) basis set for the ligand. Molecular electrostatic potential (MEP) and average local ionization energy (ALIE) surfaces were calculated to study the reactive properties of the compounds. The thermal behaviour of the compounds was determined by simultaneous thermogravimetric-differential scanning calorimetric (TG-DSC) measurements and the results were correlated to the proposed structures and to calculated MEP/ALIE surfaces. The compounds were tested in vitro for antiproliferative effects on parental (L5178Y) mouse T-cell lymphoma cells, on L5178Y transfected with pHa ABCB1/A retrovirus and for reversal of multidrug resistance (MDR) in tumor cells by flow cytometry. The preliminary measurements showed that the cobalt(III) compound was an effective inhibitor of the ABC transporter PGP drug efflux pump. The ligand was somewhat less effective, the zinc complex had a moderate inhibition activity, whereas the nickel(II) and copper(II) compounds were inactive.
- This article is part of the themed collection: Nitrogen Ligands


 Please wait while we load your content...
Please wait while we load your content...