Metal–organic framework MIL-101 supported bimetallic Pd–Cu nanocrystals as efficient catalysts for chromium reduction and conversion of carbon dioxide at room temperature†
Abstract
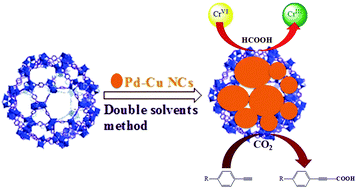
This paper reports a series of bimetallic Pd–Cu nanocrystals (NCs) supported on the zeolite-type metal–organic framework MIL-101 to yield Pd–Cu/MIL-101 nanocomposites using a double solvent method. The obtained composites, Pd–Cu/MIL-101, are 5.5–11 nm in size and are supported on the surface of MIL-101. TEM images reveal that the loading of nanocrystals is uniform across the MIL-101. These represent highly active MOF-supported metal catalysts for the reduction of Cr(VI) to Cr(III) using formic acid and the conversion of terminal alkynes into propiolic acids with CO2 at room temperature. The catalytic activities toward the reduction of Cr(VI) to Cr(III) and the conversion of terminal alkynes into propiolic acids, Pd–Cu/MIL-101 nanocomposites showed better performance, by a factor of 3–5 times faster than Pd–Cu nanocrystals. Being heterogeneous, the Pd–Cu/MIL-101 nanocomposite catalysts are also easy to handle and separate from the reaction mixture, and can be recycled five times with no loss of activity.

 Please wait while we load your content...
Please wait while we load your content...