The syntheses, structures and azo–hydrazone tautomeric studies of three triazole/tetrazole azo dyes†
Abstract
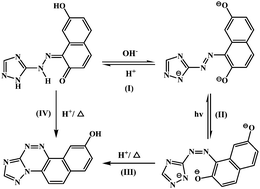
Three heterocyclic azo dyes of azotriazolyl-2,7-dihydroxynaphthalene (H3AD), azotetrazolyl-2-naphthol (H2ATN) and azotetrazolyl-2,7-dihydroxynaphthalene (H3ATD) have been synthesized and characterized by IR, Raman spectra, 1H, 13C NMR and X-ray single-crystal diffraction techniques and their azo–hydrazone tautomerism has been achieved by pH control and coordination-coupled proton transfer. UV-vis spectra have been used to monitor the pH-titration and metal-ion complex experiments for three solutions of H3AD, H2ATN and H3ATD and the analysis of their spectral data has provided a clear proof that the hydrazone form is the dominant tautomer in acidic and neutral solutions, while the azo form is the dominant one in alkaline solutions. Crystallographic data demonstrate that the neutral dyes exist as hydrazone tautomers but are changed to azo tautomers when they coordinate to metal ions. The time-dependent density functional theory (TD-DFT) calculations are used to demonstrate the UV-vis spectroscopic properties.

 Please wait while we load your content...
Please wait while we load your content...