Defining the metal binding pathways of human metallothionein 1a: balancing zinc availability and cadmium seclusion
Abstract
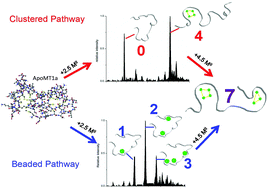
Metallothioneins (MTs) are cysteine-rich, metal-binding proteins that are found throughout Nature. This ubiquity highlights their importance in essential metal regulation, heavy metal detoxification and cellular redox chemistry. Missing from the current description of MT function is the underlying mechanism by which MTs achieve their proposed biological functions. To date, there have been conflicting reports on the mechanism of metal binding and the structures of the metal binding intermediates formed during metalation of apoMTs. The form of the metal-bound intermediates dictates the metal sequestering and metal-donating properties of the protein. Through a detailed analysis of spectral data from electrospray ionization mass spectromeric and circular dichroism methods we report that Zn(II) and Cd(II) metalation of the human MT1a takes place through two distinct pathways. The first pathway involves formation of beaded structures with up to five metals bound terminally to the 20 cysteines of the protein via a noncooperative mechanism. The second pathway is dominated by the formation of the four-metal domain cluster structure M4SCYS11via a cooperative mechanism. We report that there are different pathway preferences for Zn(II) and Cd(II) metalation of apo-hMT1a. Cd(II) binding follows the beaded pathway above pH 7.1 but beginning below pH 7.1 the clustered (Cd4Scys11) pathway begins to dominate. In contrast, Zn(II) binding follows the terminal, “beaded”, pathway at all physiologically relevant pH (pH ≥ 5.2) only following the clustered pathway below pH 5.1. The results presented here allow us to reconcile the conflicting reports concerning the presence of different metalation intermediates of MTs. The conflict regarding cooperative versus noncooperative binding mechanisms is also reconciled with the experimental results described here. These two metal-specific pathways and the presence of radically different intermediate structures provide insight into the multi-functional nature of MT: binding Zn(II) terminally for donation to metalloenzymes and sequestering toxic Cd(II) in a cluster structure.
- This article is part of the themed collection: Zinc in the Biosciences

 Please wait while we load your content...
Please wait while we load your content...