Newport Green, a fluorescent sensor of weakly bound cellular Zn2+: competition with proteome for Zn2+†
Abstract
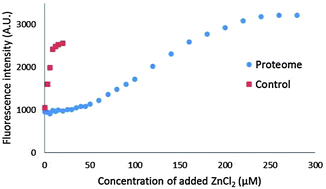
Newport Green (NPG) is a recognized sensor of cellular Zn2+ that displays fluorescence enhancement upon binding to Zn2+. Because of its modest affinity for Zn2+, the extent of its capacity to bind cellular Zn2+ is unclear. The present study investigated the range of reactivity of NPGESTER with cells, isolated (Zn)-proteome, and model Zn-proteins. The sensor accumulated in pig kidney LLC-PK1 cells and was slowly (>40 min) hydrolyzed to the fluorescent, acid form, NPGACID. The powerful, cell permeant Zn2+ chelator, N,N,N′,N′-tetrakis(2-pyridylmethyl)-ethane-1,2-diamine (TPEN) failed to quench the growing fluorescence emission, indicating that Zn–NPGACID had not formed and NPG–Zn-protein adduct species probably were not present. Furthermore, NPGACID did not bind to Zn-carbonic anhydrase or Zn-alcohol dehydrogenase, two proteins that form adducts with some other sensors. Strikingly, most of the NPGACID that had been converted from NPGESTER was detected in the extracellular medium not the cells. As a result, after cells were incubated with NPGESTER and then Zn-pyrithione to raise the internal concentration of mobile Zn2+, Zn–NPGACID was only observed in the external medium. Residual cellular NPGACID was unable to bind extra intracellular Zn2+ delivered by pyrithione. Proteome isolated from the sonicated cell supernatant was also unreactive with NPGACID. Titration of proteome or glutathione with Zn2+ in the presence of NPGACID revealed that NPGACID only weakly competes for mobile Zn2+ in the presence of these cellular components. In addition, when proteomic Zn2+ was released by a nitric oxide donor or N-ethyl-maleimide, little Zn2+ was detected by NPGACID. However, exposure to nitric oxide independently enhanced the fluorescence properties of NPGACID. Thus, the biochemical properties of NPG related to cellular Zn2+ chelation deepen the question of how it functions as a Zn2+ sensor.

 Please wait while we load your content...
Please wait while we load your content...