Boosting the electron mobility of solution-grown organic single crystals via reducing the amount of polar solvent residues†
Abstract
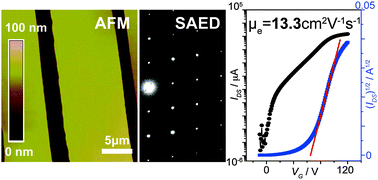
Enhancing electron transport to match with the development in hole transport is critical for organic electronics in the future. As electron motion is susceptible to extrinsic factors, seeking these factors and avoiding their negative effects have become the central challenge. Here, the existence of polar solvent residues in solution-grown single-crystals of 6,13-bis(triisopropylsilylethynyl)-5,7,12,14-tetraazapentacene is identified as a factor detrimental to electron motion. Field-effect transistors of the crystals exhibit electron mobility boosted by about 60% after the residues are removed. The average electron mobility reaches up to 8.0 ± 2.2 cm2 V−1 s−1 with a highest value of 13.3 cm2 V−1 s−1; these results are significantly higher than those obtained previously for the same molecule (1.0–5.0 cm2 V−1 s−1). Furthermore, the achieved mobility is also higher than the maximum reported electron mobility for organic materials (11 cm2 V−1 s−1). This work should greatly accelerate the advancement of organic electron-transporting materials.

 Please wait while we load your content...
Please wait while we load your content...