Design, synthesis and anticancer activity of dihydropyrimidinone–semicarbazone hybrids as potential human DNA ligase 1 inhibitors†‡
Abstract
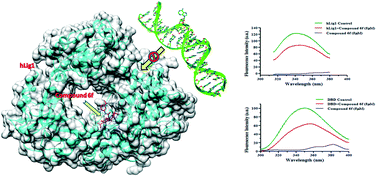
A series of new dihydropyrimidinone–semicarbazone hybrids were successfully synthesised by integrating regioselective multicomponent reaction with the pharmacophore hybridization approach. All the synthesised compounds were evaluated for their hLig1 inhibition potency and most of them were found to be good to moderately active. Out of the tested derivatives, compound 6f showed selective anti-proliferative activity against HepG2 cells in a dose-dependent manner with an IC50 value of 10.07 ± 1.2. It also reduced cell survival at ≤20 μM concentration. Further, analysis of treated HepG2 cell lysates by western blot assay showed increased γ-H2AX levels and upregulation of p53, leading to apoptosis. In silico docking results explain the binding modes of compound 6f to the DNA-binding domain of hLig1 enzyme thereby preventing its nick sealing activity. In addition, the favourable pharmacokinetic properties suggest that this new class of hLig1 inhibitors could be promising leads for further drug development.


 Please wait while we load your content...
Please wait while we load your content...