Synthesis and in vitro evaluation of the antitumor potential and chemo-sensitizing activity of fluorinated ecdysteroid derivatives†‡
Abstract
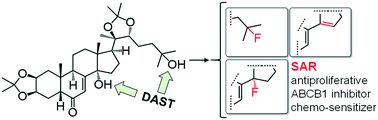
Efflux pumps, like the ABCB1 transporter, play an important role in the chemo-resistance of various tumors and particularly of cancer stem cells. We have previously reported the chemo-sensitizing activity of apolar ecdysteroid derivatives on cancer cell lines of various origin and sensitivity to chemotherapeutics. Herein we report the preparation of three fluorinated derivatives and a dehydrated byproduct from 20-hydroxyecdysone 2,3;20,22-diacetonide (1) through diethylaminosulfur trifluoride (DAST)-mediated fluorination. Complete NMR assignment of the products is provided. In vitro bioactivity testing was performed on four human breast cancer cell lines, a neuroblastoma, and a mouse lymphoma cell line and its counterpart expressing the human ABCB1 efflux transporter. Fluorination increased the ABCB1 inhibitory effect of the compounds but had little effect on their limited antiproliferative action, which was, however, markedly increased by a Δ14,15 double bond. Compound 5, a new 14,25-difluoro analog of 1, exerted higher chemo-sensitizing activity to doxorubicin as compared to its parental compound.


 Please wait while we load your content...
Please wait while we load your content...