Synthesis and antiproliferative activity of novel hybrid 3-substituted polyhydroquinoline-fatty acids†‡
Abstract
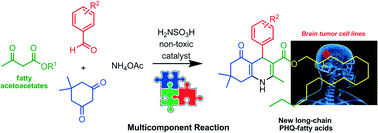
This work describes the synthesis of new long-chain fatty acid polyhydroquinoline derivatives (hybrid PHQ-fatty acids) using a Hantzsch four-component reaction without the use of metals and catalyzed by non-toxic acids. The PHQ-fatty acids were synthesized from fatty β-ketoesters in the presence of sulfamic acid (H2NSO3H) in good yields (70–81%). Following the synthesis, for the first time, the in vitro antiproliferative activity of a series of long-chain PHQ-fatty acids was investigated in a glioma cell line (C6 rat malignant GBM cell line). Notably, all of the tested compounds were active against this cell line. Superior activity was observed for PHQ with a hydroxyl group on the aromatic ring and a stearic fatty acid chain, reducing the cell viability by ca. 40% at 5 μM.

 Please wait while we load your content...
Please wait while we load your content...