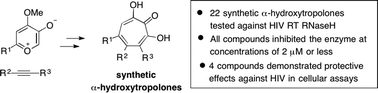
Abstract
HIV reverse transcriptase-associated ribonuclease H activity is a promising enzymatic target for drug development that has not been successfully targeted in the clinic. While the α-hydroxytropolone-containing natural products β-thujaplicinol and manicol have emerged as some of the most potent leads described to date, structure–function studies have been limited to the natural products and semi-synthetic derivatives of manicol. Thus, a library of α-hydroxytropolones synthesized through a convenient oxidopyrylium cycloaddition/ring-opening sequence have been tested in in vitro and cell-based assays, and have been analyzed using computational support. These studies reveal new synthetic α-hydroxytropolones that, unlike the natural product leads they are derived from, demonstrate protective antiviral activity in cellular assays.

 Please wait while we load your content...
Please wait while we load your content...