Synthesis and molecular docking studies of a new series of bipyrazol-yl-thiazol-ylidene-hydrazinecarbothioamide derivatives as potential antitubercular agents†‡
Abstract
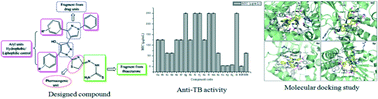
Various substituted 2-(2-(5-(3/4-substituted phenyl)-4-hydroxy-3′-(3/4-substituted phenyl)-1′-phenyl-1H,1′H-[3,4′-bipyrazol]-1-yl)thiazol-4(5H)ylidene) hydrazinecarbothioamide derivatives have been synthesized in good yields by an efficient method. The synthesized compounds (6a–r) were evaluated for their in vitro antitubercular activity against the Mycobacterium tuberculosis (MTCC 300) strain. Compounds 6o (MIC-3.90 μg mL−1), 6p (MIC-3.90 μg mL−1), and 6q (MIC-7.81 μg mL−1), exhibited significant activity against Mycobacterium tuberculosis. The molecular docking studies revealed an interesting binding profile with very high receptor affinity.

 Please wait while we load your content...
Please wait while we load your content...