Progress in the development of small molecules as new human A3 adenosine receptor ligands based on the 3-thiophenylcoumarin core†‡
Abstract
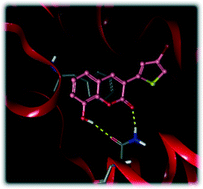
Adenosine receptors (AR) are GPCRs involved in several biochemical processes. Agents able to selectively modulate the activity of these receptors represent promising multifunctional agents to delay or slow the progression of a large range of diseases. Here, differently substituted 3-thiophenylcoumarins are described that exert affinity towards human AR subtypes. Among the compounds synthesized, the 3-(4-bromothiophenyl) derivative 11 showed the highest affinity and selectivity for the hA3 AR (Ki = 740 nM). Interestingly, the current study revealed that small structural changes in this scaffold allow modulating the AR affinity, suggesting that this scaffold has desirable properties for the development of promising classes of hA1, hA2A, and/or hA3 AR ligands. Further docking calculations in the hA3 AR identified the hypothetical binding mode of the most active and selective compounds. In addition, theoretical evaluation of some physicochemical properties highlighted the potential of these compounds as drug candidates. The data so far acquired is the first step for further optimization of these 3-thiophenylcoumarins as hA3 AR selective ligands.

 Please wait while we load your content...
Please wait while we load your content...