The role of transporter ectodomains in drug recognition and binding: phlorizin and the sodium–glucose cotransporter†
Abstract
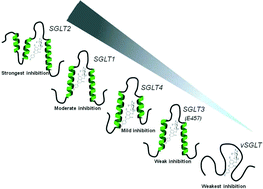
This article reviews the role of segments of SLCs located outside the plasma membrane bilayer (ectodomains) using the inhibition of SGLTs (SLC5 family) by the aromatic glucoside phlorizin as a model system. Phlorizin has been the lead substance for the development of SGLT2 (SLC5A2) inhibitors that have been introduced recently for the treatment of type 2 diabetes. Using mainly biophysical methods, it is shown that three ectodomains form well-defined substructures, such as short helices, that are arranged in a vestibule and undergo significant conformational changes during binding of phlorizin. From these data, tentative structures of inhibitor/ectodomain complexes are derived by molecular modeling. The ectodomains provide an additional binding site for aglucones, which cooperates with the binding site for the sugar moiety of phlorizin in the sugar translocation pathway, buried inside the membrane. They play a significant role in determining the specificity, selectivity, and affinity of sugar transport inhibitors and might even explain the difference in sensitivity of various members of the SGLT family, located in different tissues and organs of the human body. Similar binding modes are suggested for several other SLCs, in particular the monoamine transporter (SLC6 family), that belong to the sodium/neurotransmitter cotransporter family.

 Please wait while we load your content...
Please wait while we load your content...