Competitive profiling of celastrol targets in human cervical cancer HeLa cells via quantitative chemical proteomics†
Abstract
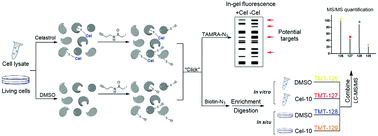
Celastrol, isolated from the traditional Chinese medicinal herb Tripterygium wilfordii Hook. f. (Thunder God's Vine), has been used to treat cancer, chronic inflammatory, autoimmune and other human diseases. However, to date, the protein targets and the mechanism of action of celastrol have remained elusive. In this study, we find that celastrol can react with protein thiols in a unique covalent and reversible manner, while protein denaturing disrupts the interaction. Through a competitive chemoproteomics approach utilizing a cysteine-targeting activity-based probe, we report the proteome-wide quantitative profiling of cellular targets of celastrol in human cervical cancer HeLa cells. Representative targets are further validated via in vitro binding experiments and/or enzymatic activity assays. Bioinformatics analysis results suggest that celastrol exerts its numerous therapeutic effects through interaction with promiscuous proteins involved in various biological processes and cellular pathways.


 Please wait while we load your content...
Please wait while we load your content...