Molecular dynamics studies on the DNA-binding process of ERG†
Abstract
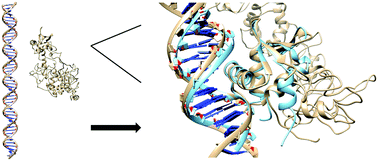
The ETS family of transcription factors regulate gene targets by binding to a core GGAA DNA-sequence. The ETS factor ERG is required for homeostasis and lineage-specific functions in endothelial cells, some subset of haemopoietic cells and chondrocytes; its ectopic expression is linked to oncogenesis in multiple tissues. To date details of the DNA-binding process of ERG including DNA-sequence recognition outside the core GGAA-sequence are largely unknown. We combined available structural and experimental data to perform molecular dynamics simulations to study the DNA-binding process of ERG. In particular we were able to reproduce the ERG DNA-complex with a DNA-binding simulation starting in an unbound configuration with a final root-mean-square-deviation (RMSD) of 2.1 Å to the core ETS domain DNA-complex crystal structure. This allowed us to elucidate the relevance of amino acids involved in the formation of the ERG DNA-complex and to identify Arg385 as a novel key residue in the DNA-binding process. Moreover we were able to show that water-mediated hydrogen bonds are present between ERG and DNA in our simulations and that those interactions have the potential to achieve sequence recognition outside the GGAA core DNA-sequence. The methodology employed in this study shows the promising capabilities of modern molecular dynamics simulations in the field of protein DNA-interactions.

 Please wait while we load your content...
Please wait while we load your content...