Spectroscopic evidence for the role of a site of the di-iron catalytic center of ferritins in tuning the kinetics of Fe(ii) oxidation†
Abstract
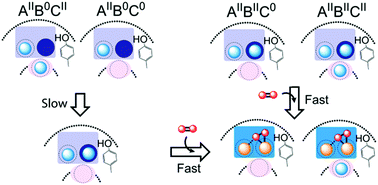
Ferritin is a nanocage protein made of 24 subunits. Its major role is to manage intracellular concentrations of free Fe(II) and Fe(III) ions, which is pivotal for iron homeostasis across all domains of life. This function of the protein is regulated by a conserved di-iron catalytic center and has been the subject of extensive studies over the past 50 years. Yet, it has not been fully understood how Fe(II) is oxidized in the di-iron catalytic center and it is not known why eukaryotic and microbial ferritins oxidize Fe(II) with different kinetics. In an attempt to obtain a new insight into the mechanism of Fe(II) oxidation and understand the origin of the observed differences in the catalysis of Fe(II) oxidation among ferritins we studied and compared the mechanism of Fe(II) oxidation in the eukaryotic human H-type ferritin (HuHF) and the archaeal ferritin from Pyrococcus furiosus (PfFtn). The results show that the spectroscopic characteristics of the intermediate of Fe(II) oxidation and the Fe(III)-products are the same in these two ferritins supporting the proposal of unity in the mechanism of Fe(II) oxidation among eukaryotic and microbial ferritins. Moreover, we observed that a site in the di-iron catalytic center controls the distribution of Fe(II) among subunits of HuHF and PfFtn differently. This observation explains the reported differences between HuHF and PfFtn in the kinetics of Fe(II) oxidation and the amount of O2 consumed per Fe(II) oxidized. These results provide a fresh understanding of the mechanism of Fe(II) oxidation by ferritins.


 Please wait while we load your content...
Please wait while we load your content...