A microfluidic study of liquid–liquid extraction mediated by carbon dioxide†
Abstract
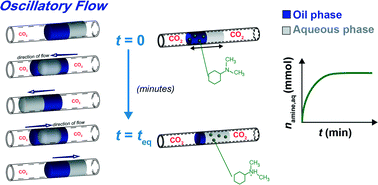
Liquid–liquid extraction is an important separation and purification method; however, it faces a challenge in reducing the energy consumption and the environmental impact of solvent (extractant) recovery. The reversible chemical reactions of switchable solvents (nitrogenous bases) with carbon dioxide (CO2) can be implemented in reactive liquid–liquid extraction to significantly reduce the cost and energy requirements of solvent recovery. The development of new effective switchable solvents reacting with CO2 and the optimization of extraction conditions rely on the ability to evaluate and screen the performance of switchable solvents in extraction processes. We report a microfluidic strategy for time- and labour-efficient studies of CO2-mediated solvent extraction. The platform utilizes a liquid segment containing an aqueous extractant droplet and a droplet of a solution of a switchable solvent in a non-polar liquid, with gaseous CO2 supplied to the segment from both sides. Following the reaction of the switchable solvent with CO2, the solvent becomes hydrophilic and transfers from the non-polar solvent to the aqueous droplet. By monitoring the time-dependent variation in droplet volumes, we determined the efficiency and extraction time for the CO2-mediated extraction of different nitrogenous bases in a broad experimental parameter space. The platform enables a significant reduction in the amount of switchable solvents used in these studies, provides accurate temporal characterization of the liquid–liquid extraction process, and offers the capability of high-throughput screening of switchable solvents.

 Please wait while we load your content...
Please wait while we load your content...