Vinculin head–tail interaction defines multiple early mechanisms for cell substrate rigidity sensing†
Abstract
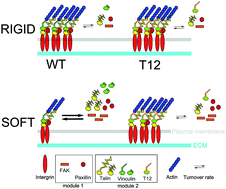
Rigidity sensing is a critical determinant of cell fate and behavior but its molecular mechanisms are poorly understood. Focal adhesions (FAs) are complexes that anchor cells to the matrix. Among their components, vinculin undergoes an auto-inhibitory head–tail interaction that regulates the recruitment of, and interactions with its partners in a force-dependent manner. It is unknown, however, whether this mechanism is involved in substrate rigidity sensing. Here, we use a range of quantitative fluorescence microscopies on live human Mesenchymal Stem Cells to address this question. We identify two distinct rigidity-sensing molecular modules in FAs, one of which involves vinculin and talin, is regulated by vinculin head–tail interaction, and targets cell morphology. Vinculin and talin are recruited independently in a rigidity-dependent manner to FAs where they directly interact in a rigidity-independent stoichiometry at a site proximal to talin head. Vinculin head–tail interaction is required on soft substrates to destabilize vinculin and talin in FAs, and to allow hMSCs branching. Another module involves paxillin and FAK, which soft substrates also destabilize, but independently of vinculin head–tail interaction. This multi-modularity may be key to allow a versatile response to complex biomechanical cues.

 Please wait while we load your content...
Please wait while we load your content...