A metal free reduction of aryl-N-nitrosamines to the corresponding hydrazines using a sustainable reductant thiourea dioxide†
Abstract
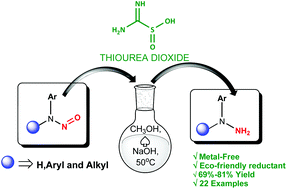
Reduction of aryl-N-nitrosamines to the corresponding hydrazines is reported under metal free conditions using a sustainable industrial reductant thiourea dioxide (TDO). The reaction takes place under mild conditions in an aqueous medium to provide good to excellent yields of the desired products. Sensitive functional groups such as olefins, alkynes and aryl halides were found to be stable during the reduction while a wide range of aryl-N-nitrosamines were successfully converted to the desired hydrazines. One-pot synthesis of arylhydrazines from their corresponding secondary amines via an N-nitrosamine intermediate was established.

 Please wait while we load your content...
Please wait while we load your content...